Details of the Drug
General Information of Drug (ID: DMOUC09)
| Drug Name |
Norepinephrine
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
norepinephrine; L-Noradrenaline; Levarterenol; 51-41-2; L-Norepinephrine; Levophed; (-)-Norepinephrine; L-arterenol; (R)-Noradrenaline; (R)-Norepinephrine; Levonor; Levonorepinephrine; Adrenor; Aktamin; Levonoradrenaline; Sympathin E; (R)-(-)-Norepinephrine; 4-[(1R)-2-amino-1-hydroxyethyl]benzene-1,2-diol; Noradrenalin; Norepirenamine; norepinephrinum; Noradrenalinum; Levoarterenol; Norartrinal; Nor-Epirenan; L-3,4-dihydroxyphenylethanolamine; Levarterenolo; Noreinefrina; Norepinefrina; Norepinephrinum; Levarterenolo [DCIT]; Nor adrenalin; Noradrenalina [Italian]; Norepinephrine Noradrenalin; LT03330026; ALBB-006229; Nor adrenalin (TN); Noradrenaline (JP15); Noreinefrina [INN-Spanish]; Norepinephrine (INN); Norepinephrine [INN:JAN]; Norepinephrinum [INN-Latin]; Norepinephrine l-Tartrate (1:1); D-(-)-Noradrenaline; D53D5E3A-2360-4CA9-8031-6C2CD4062FD5; L-alpha-(aminomethyl)-3,4-dihydroxybenzyl alcohol; L-1-(3,4-Dihydroxyphenyl)-2-aminoethanol; L-2-Amino-1-(3,4-dihydroxyphenyl)ethanol; (-)-(R)-Norepinephrine; (-)-Arterenol free base; (-)-alpha-(Aminomethyl)protocatechuyl alcohol; (R)-4-(2-Amino-1-hydroxyethyl)-1,2-benzenediol; 1,2-Benzenediol, 4-(2-amino-1-hydroxyethyl)-, (R)-(9CI); 4-[(1R)-2-Amino-1-hydroxyethyl]-1,2-benzenediol; Ecteinascidins
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Vasoconstrictor Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
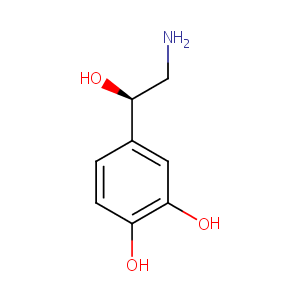 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 169.18 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1.2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Norepinephrine (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
